Mitigating Batrachochytrium salamandrivorans
in Europe

Morphology & Thermal Preference
Batrachochytrium salamandrivorans (Bsal) is a fungus in the phylum Chytridiomycota and the order Rhizophydiales. Like B. dendrobatides, Bsal has demonstrated two main life stages: a motile zoospore with a single posteriorly directed flagellum and a mature thallus, containing asexual zoospores, called zoosporangium (Van Rooij et al. 2015).
In vitro, Bsal produces motile zoospores from colonial or monocentric thalli. Colonial thalli contain more than one sporangium while monocentric thalli contain only one sporangium. Two principal distinguishing morphological features of Bsal in culture are the development of germ tubes from encysted zoospores and the presence of more colonial thalli than can be observed in Bd cultures.(Martel et al. 2013, Van Rooij et al. 2015)
Recently Stegen et al. 2017 described an additional infectious form of Bsal spore, a robust encysted spore with the ability to persist in the environment for extended periods. This encysted spore is produced both in vivo and vitro and has its own specific infectious, transmission and persistence strategy which contribute to its durability and hardy nature.
Bsal grows optimally between 10°C - 15°C, however, it also grows, albeit slowly at 5°C. The death of Bsal occurs at ≥ 25°C. (Martel et al. 2013)

(Martel et al. 2013)
(Martel et al. 2013)
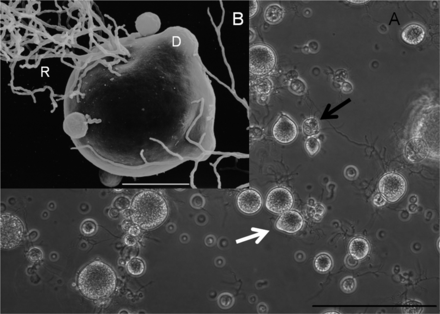
In vitro culture of B. salamandrivorans in TGhL broth at 15 °C. (A) Monocentric thalli predominate, with the rare presence of colonial thalli (black arrow). Sporangia develop discharge tubes (white arrow) to release zoospores (Scale bar, 100 μm.) (B) Scanning electron microscopic image of a mature sporangium with rhizoids (R), discharge tubes (D), and germ tube formation (arrow) (Scale bar, 10 μm.)
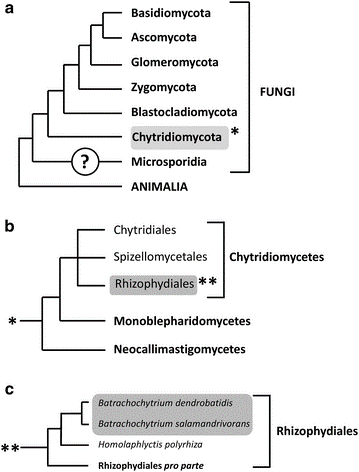
Phylogeny and classification of the genus Batrachochytrium.
Cladogram showing the taxonomic position of Batrachochytrium dendrobatidis and Batrachochytrium salamandrivorans within the fungal kingdom (a), the phylum Chytridiomycota (b) and order of the Rhizophydiales (c). The position of the Microsporidia remains uncertain. Branch lengths are not proportional to genetic distances. The topology is derived from Martel et al. [13], Longcore et al. [16] and Hibbett et al.
Host Range
Bsal is pathogenic for most western Palearctic salamander and newt taxa and is considered a major threat to the region’s biodiversity (Martel et al., 2013; Spitzen van der Sluijs et al., 2016). Salamanders can be resistant (no infection, no disease), tolerant (infection in absence of disease), moderately susceptible (infection resulting in clinical disease with possibility of subsequent recovery) or highly susceptible (infection resulting in lethal disease). Infection experiments demonstrated that frogs and toads are not susceptible to the disease, but can act as infectious carriers (Stegen et al. 2017).

Pathogenesis
Until recently very little was known about the pathogenesis of B salamandrivorans. However, Stegen et al 2017 and Farrer et al. 2017 have recently, through novel discoveries, contributed to the elucidation of Bsal's host pathogen-interraction and virulence.
Stegen et al. 2017, revealed that as part of its deadly infection arsenal, both in vitro and in vivo, Bsal has two infectious forms, a motile zoospore and an encysted spore. The encysted Bsal spore has separate infection, dissemination and persistence methods which make it more resistant to predation and allow its persistence in the environment and its passive spread by several vectors. While zoospores swim to potential hosts, Stegen et al. 2017 described encysted spores as floating at the surface of the water with the possibility of adhering to amphibian skin and to the feet of waterfowl resulting in their widespread dissemination. Encysted spores have been shown to also remain viable in aquatic environments for at least 31 days and mounted better resistance to zooplankton predation than the Bsal zoospores. Soils contaminated with Bsal by infected amphibians yielded positive results for Bsal DNA even after 200 days and infection via contaminated soil occurred up to 48 hours after the soil was in contact with the initial source of contamination.
Anurans and less susceptible amphibians are also implicated in the persistence of Bsal as they are able to resist the disease but act as reservoirs of infection for other more susceptible amphibian species.
Stegen et al. 2017 further stated that the outcome of Bsal infection in the host was not determined by fungal load or environmental temperature and that both low and high doses of Bsal were able to produce disease in highly susceptible species such as the fire salamander (Salamandra salamandra).
In addition, even after several rounds of non-lethal infection, Bsal failed to provoke an immune response in its amphibian hosts. Farrer et al. 2017 suggest that one of Bsal's unique gene families may be responsible for the absence of a protective host immune response when confronted by this pathogen.
Stegen et al. 2017 expressed concern that in regard to mitigation of the disease, all these factors collude to make the eradication or even in situ control of Bsal-mediated chytridiomycosis extremely difficult and maybe even impossible.
Farrer et al. 2017 investigated the genomes of Bd and Bsal and reported that Bsal's genome is 32.6 Mb and is more extensive and complex while Bd's is smaller at 23.7 Mb. The authors discovered several genes in the genome of both pathogens which they compared with the genomes of the saprophytic members of the fungal phylum Chytridiomycota and credit both Bd's and Bsal's pathogenicity to an incredible extension of protease and cell wall gene families. The evolution of Batrachochytrium species to produce infection in amphibians is correlated to the incorporation of genes coding for putative virulence factors which are unique to the Batrachochytrium genus or specific to the Bd or Bsal genome. They identified gene-families peculiar to Bd or Bsal which were deemed responsible for their disparate infection modus operandi.
The gene expression of Bd and Bsal during the process of infection or growth in culture were compared. Of the 550 chytrid genes which were significantly upregulated, 327 were unique to Bsal while 43 were unique to Bd and 44 were unique to the genus Batrachochytrium. Several of the expanded and lineage-specific proteases were upregulated in the course of in vivo infection of the newt T. wenxianensis. Also, approximately half of the Batrachochytrium, Bd and Bsal genes were secreted. As these or similarly functioning proteins were not discovered in the saprobic chytrids and the fact that the transcription of these proteins increases during colonization, imply that the up-regulation during this time demonstrates an individualized Bsal-host interaction.
Farrer et al. 2017 suggest that among these upregulated genes there is a likelihood that these include virulence factors for host-specific colonization. Some of these are the M36 metalloproteases (known to function in host colonization in other pathogens) and Tribes 1 and 4 which are two large families of secreted and highly expressed proteins. Bsal has 110 M36 metalloproteases while Bd has 35, both of which are much higher numbers of metalloproteases than can be found in the related saprobes Spizellomyces puncatatus and Homolaphlyctis polyrhiza, with the former containing 2 and the latter 3. Some of these M36 metalloproteases are specific to the genus Batrachochytrium possibly furnishing them with the ability to adapt to vertebrate hosts while others are specific to Bsal or Bd playing a role in the individual characteristics defining the species and their individual pathogeneses.
Farrer et al. 2017 also noted the decreased activity of the G1M36 metalloprotease in Bsal zoospores but indicated an increase of activity of this protease in the Bsal sporangia. They suggest that this may be associated with a subsequent important function in pathogenesis including traversing the sporangial wall and disseminating to adjacent host tissue.
Another protein family which is present in the pathogenic chytrids more abundantly than in the independently existing chytrids contains multiple copies of a domain called CBM18. These proteins are targeted to the cell surface or extracellular space and the suggested function of this domain is the binding of chitin. There is marked truncation of the CBM18s in Bsal compared to those in Bd. In addition, those in Bsal contain fewer CBM18 domains than Bd. Farrer et al. 2017 surmise that this protein family functions in fungal attachment to the host skin and in diminishing the detection of chitin by the host immune system.
Repeat-rich sequences correlate with the expansion of the M36 metalloproteases and CBM18 genes. Bd and Bsal contain 17% and 16% of repeat-rich sequences respectively compared to the non-pathogenic Spizellomyces puncatatus and Homolaphlyctis polyrhiza which contain 3.7% and 4.5% respectively.
Clinical Signs
Metamorphosed urodelans who are Infected with Bsal display apathy, anorexia, ataxia, skin erosions and ulcerations all over the body. They also show signs of lethargy. (Van Rooij et al. 2015, Martel et al. 2013)
It is important to note that the signs of Bsal infection can be easily missed or remain undetected as the lesions do not usually appear till the final stage of the disease. (Martel et al. 2013)
Pathology
In chytridiomycosis caused by Bsal there are abundant skin erosions in the epidermis which sometimes can be viewed macroscopically. These are caused by the presence of numerous intracellular colonial thalli. Also, microscopically, necrosis of the adjacent keratinocytes is visible, (Van Rooij et al. 2015). Martel et al 2013 also described the presence of keratinocytes with eosinophilic erosions and marginated nuclei at the periphery of the erosions. Martel et al 2013 further indicated that each of these keratinocytes contained one centrally located thallus with most being colonial thalli. In addition, throughout the skin, immediately beneath the damaged keratin layer, were small foci of keratinocytes. These keratinocytes also demonstrated almost identical eosinophilic necrosis, marginated nuclei and centrally located colonial thalli.



(Martel et al. 2013)
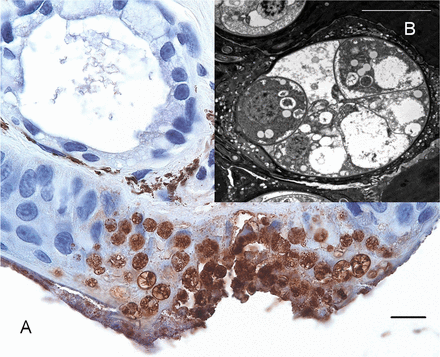
Microscopy of the skin of a fire salamander that died due to infection with B. salamandrivorans. (A) Immunohistochemical staining of a 5-µm skin section. Intracellular colonial thalli abound throughout all epidermal cell layers and are associated with erosive lesions. (B) Transmission electron microscopy picture of an intracellular colonial thallus of B. salamandrivorans inside a keratinocyte
Macroscopic view of lesions on S. salamandra skin
Diagnosis
Bsal can be diagnosed by a number of methods including clinical presentation, microscopic observation of skin scrapings, histology/histopathology, Polymerase Chain Reaction (PCR), and quantitative Polymerase Chain Reaction (qPCR) .While some literature has stated that Bsal and Bd can be diagnosed based on the characteristics of the lesions produced in the host by each pathogen, neither Bsal nor Bd produces lesions that are pathognomonic. This means that there cannot be confirmed diagnosis of Bsal or Bd infection based solely on the types of lesions they generate in their amphibian hosts. The skin lesions produced in the host should be used in conjunction with other more reliable diagnostic tests.
Diagnosis of Bsal by histology/histopathology relies on detecting the fungus in amphibian skin based on chytrid morphology. Abundant intracellular colonial thalli in the epidermis and necrosis of the adjacent keratinocytes can be observed in positive amphibian skin samples. These microscopically observed lesions are linked to the deep ulcerations and erosions observed in the infected amphibian's skin (Martel et al. 2013). While histology and histopathology are capable of detecting Bsal's presence in amphibian skin, there are important limitations of these methods. One limitation is that both these methods require high levels of expertise and experience in order to correctly diagnose Bsal infection. Another limitation is that the diagnostic efficacy of histology/histopathology is proportional with fungal load. Therefore, in cases of low fungal load, the probability of detection decreases.
Currently the most reliable and widely used method for Bsal detection is quantitative Polymerase Chain Reaction (qPCR), a molecular diagnostic method which allows not only the detection of Bsal but also the quantification of Bsal DNA present in the sample. This is an advantage over regular PCR where the presence or absence of the pathogen can be determined but fungal load cannot be quantified. The sensitivity and specificity of this method makes it superior to the previously described as it is able to detect 0.1 Genomic Equivalents (GE) of this pathogen per PCR. This allows for the detection of Bsal even in cases of subclinical infection. The efficacy of this method in detecting less than 1 GE of this pathogen makes it an extremely appropriate diagnostic method for environmental screening and diagnosis of Bsal. (Blooi et al. 2013).
Blooi et al. 2013 also describe a PCR protocol (Duplex Real-Time PCR) which allows for the simultaneous detection and quantification of both Bd and Bsal in amphibian samples.
Treatment
Exposing infected amphibians to temperatures of 25°C for a 10-day period will result in the death of Bsal and the healing of associated lesions as the pathogen’s optimal temperature range is between 10°-15°C. This is of course taking into consideration the clinical stage of the disease and the amphibians' thermal tolerance. (Martel et al. 2013, Blooi et. at. 2015)
Blooi et al. 2015 also described a treatment protocol of a combination of Voriconazole 12.5 µg/ml, Polymyxin E 2000 IU/ml and temperature of 20°C which cleared the infection in infected salamanders in 10 days.
Prevention
There is currently no record in the literature of any vaccines developed as prophylaxis against Bsal. According to Garner et al. 2016, the development of an efficacious vaccine would be labour-intensive and extremely expensive. Recent information from Stegen et al. 2017 described a lack of protective immune response in salamanders and that, even after repeated infection, their resistance to Bsal did not increase. This finding has negative implications for vaccination and development of immunity in infected populations as possible mitigation measures. However, Bsal research is still considered to be in it's infancy. There remains much more to be elucidated on the immunogenetics of susceptible host species and the biology and ecology of the pathogen itself which will determine the feasibility of imminent efficacious prevention strategies.
